How to Improve Diversity and Inclusion in Recruitment for Clinical Trials
How to Improve Diversity and Inclusion in Recruitment for Clinical Trials
Clinical trials are essential for advancing medicine, but they can only be truly effective if they represent the diverse populations who will use the treatments. Unfortunately, many groups remain underrepresented in research, including ethnic minorities, women, older adults, and individuals from rural or low-income communities. Lack of diversity in clinical trials can lead to gaps in data, limiting how applicable results are to real-world patients.
So, how can researchers, sponsors, and organizations improve diversity and inclusion in recruitment? Here are some key strategies:
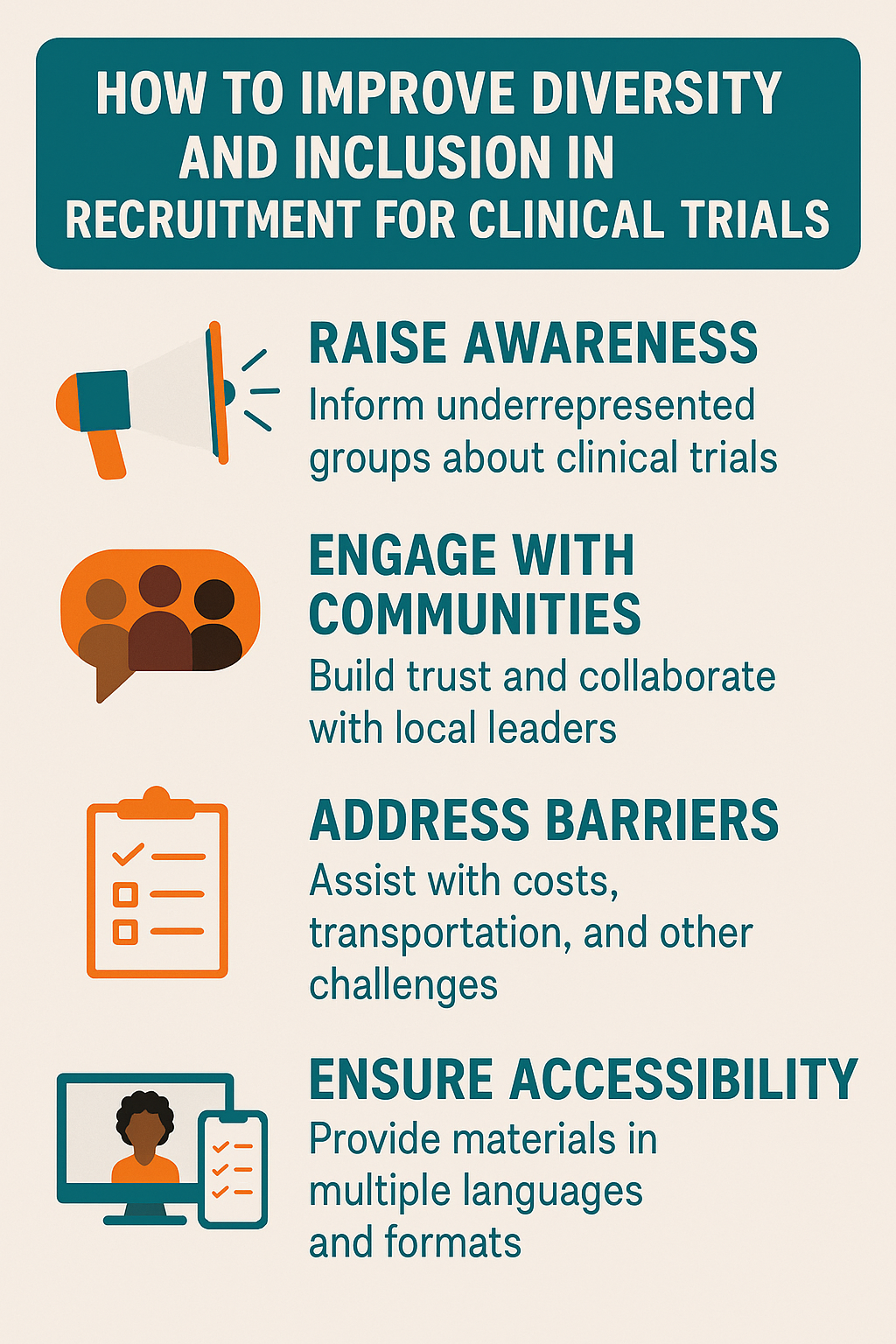
1. Address Barriers to Participation
Many individuals face barriers such as lack of access to trial sites, financial limitations, or distrust of the medical system. Offering transportation support, flexible visit schedules, childcare, or stipends can make participation more feasible. Building trust requires transparency about trial safety, benefits, and risks.
2. Simplify Eligibility Criteria
Strict inclusion/exclusion criteria can unintentionally exclude diverse populations. For example, requiring participants to be free of chronic conditions often eliminates older adults or people from disadvantaged groups. Reviewing and adjusting eligibility criteria can help broaden access without compromising safety.
3. Engage Community Leaders and Advocacy Groups
Partnerships with local leaders, patient advocacy groups, and cultural organizations can help bridge trust gaps. Community-based recruitment fosters credibility and ensures the message resonates with the intended audience.
4. Use Culturally Relevant Communication
Educational materials should reflect the language, culture, and values of the communities being recruited. Translation services, multilingual staff, and culturally tailored outreach campaigns can improve understanding and participation.
5. Leverage Technology and Decentralized Trials
Leverage Technology and Decentralized Trials
Virtual or decentralized clinical trials reduce the burden of traveling to research sites. Digital platforms, wearables, and telemedicine can make it easier for people in rural or underserved areas to participate. This approach is especially powerful for reaching populations who traditionally face access barriers.
6. Train Research Staff in Cultural Competence
Researchers and clinical staff should receive training on unconscious bias, cultural awareness, and inclusive communication. When participants feel respected and understood, they are more likely to enroll and remain engaged.
7. Monitor and Report Diversity Metrics
Tracking participant demographics throughout the recruitment process allows sponsors to identify gaps and adjust strategies. Publicly sharing diversity data also increases accountability and encourages continuous improvement.
Conclusion
Improving diversity and inclusion in recruitment is not just an ethical responsibility—it’s a scientific necessity. By ensuring that clinical trials reflect the populations who will use new treatments, we build stronger, safer, and more effective healthcare solutions. Through community engagement, cultural sensitivity, and innovative trial designs, researchers can create a more inclusive future for clinical research.
How to Improve Diversity and Inclusion in Recruitment for Clinical Trials infographics